Orally Inhaled &
Nasal Drug Products
Inhaled therapies are a major focus of pharmaceutical innovation, providing a platform to deliver an increasing array of drug products and treat an expanding range of acute and chronic conditions.
For companies developing Orally Inhaled and Nasal Drug Products (OINDPs), MOD3 Pharma provides critical support at Phase 1 & 2 through our end-to-end clinical manufacturing services, which are provided in concert with Aptar Pharma’s market-leading device platforms.
With experience of working with biologics and peptides, we guide the development of inhalable formulations in both liquid or powder form, managing all stages of the process through to device filling and cGMP manufacture. This provides our partners with a fully validated pathway for OINDPs.
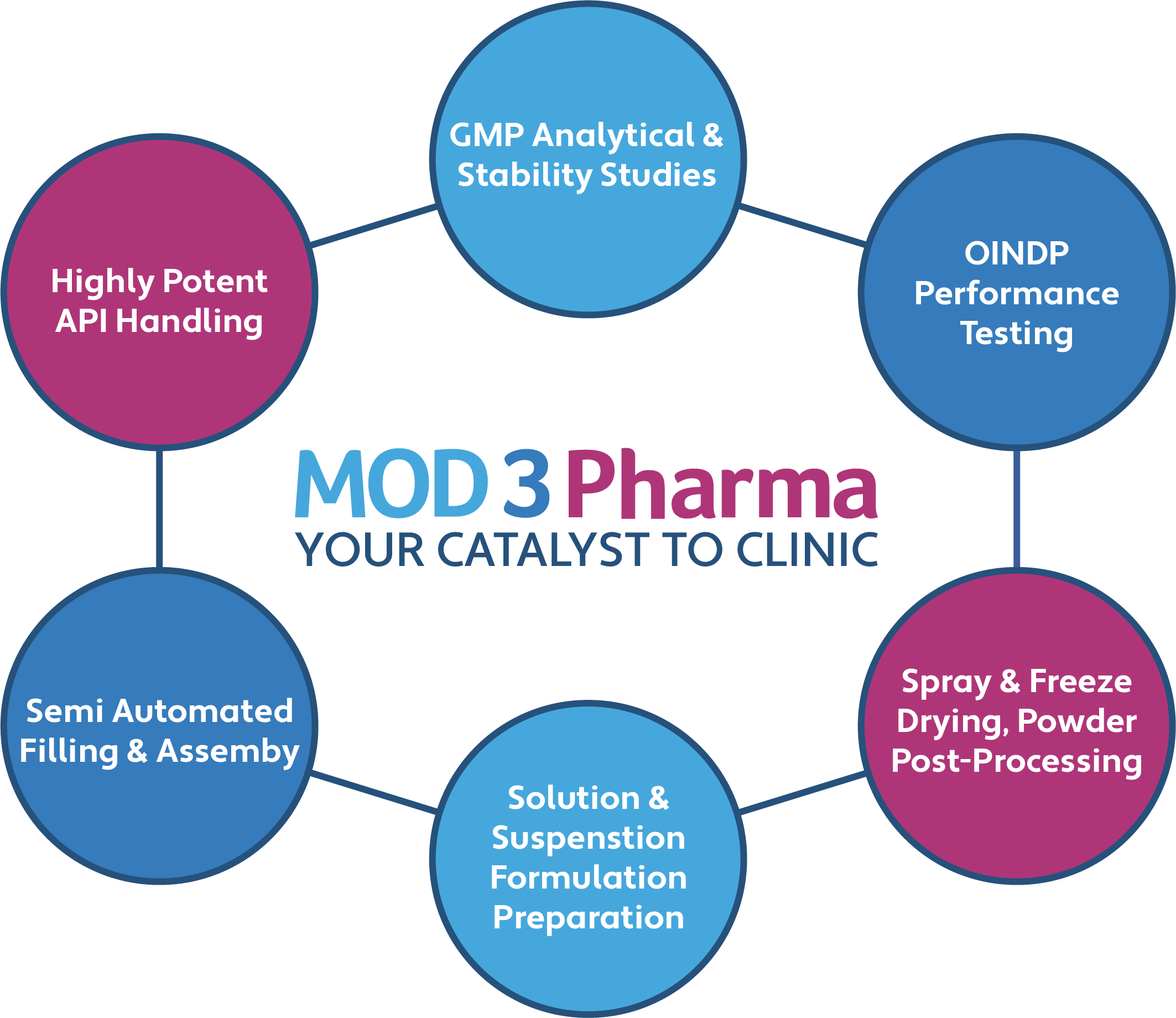
A powerful blend of drug and device expertise:
- Highly Potent API (HPAPI) handling and containment
- Extensive capabilities in the development and handling of biologics
- Analytical method development, verification, qualification and validation for dry powder inhalers, nasal powders, and nasal spray formulations
- cGMP manufacturing of nasal medicine clinical trial materials (CTM), including spray drying, freeze drying, powder post-processing and device filling and assembly
- cGMP batch release and ICH stability studies, including OINDP product performance testing
- Semi-automated, commercially representative device filling and assembly lines
- Process development and validation for technology transfer to CMO
HIGHLIGHTS OF CAPABILITIES
- CDMO intranasal product, inhalation powder CDMO,
inhalation drug delivery CDMO - 32,000 sq ft cGMP facility in New Jersey, USA
- State of the art clinical manufacturing facilities
- Extensive experience in biologics and peptides
- Inhalation drug development, dry powder inhaler CMC, inhaled and nasal product CMC
- GMP manufacturing dry powder, intranasal drug product, DPI and inhalation product testing